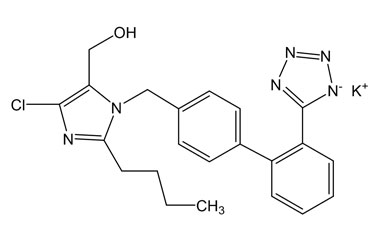
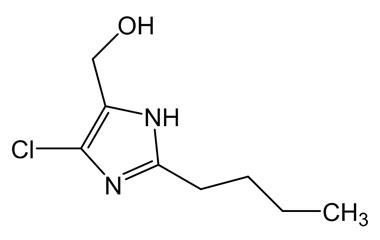
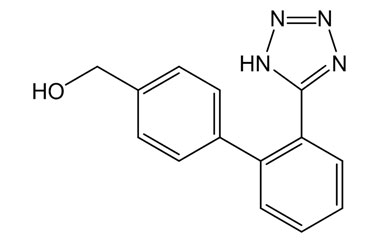
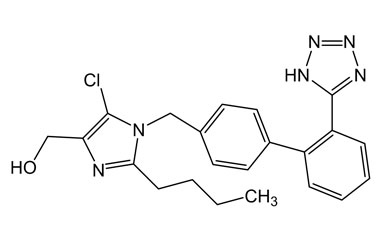
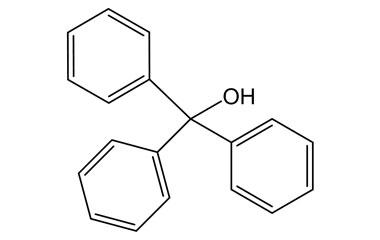
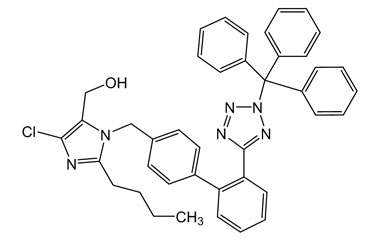
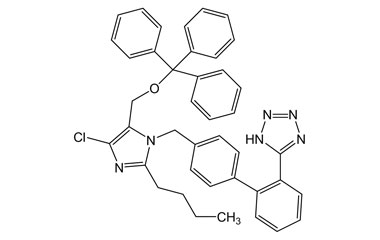
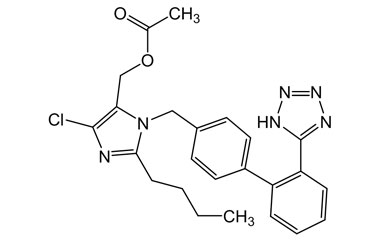
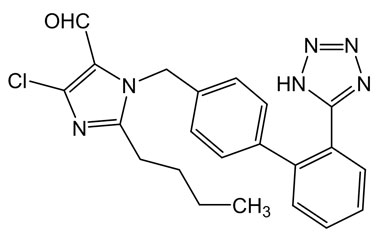
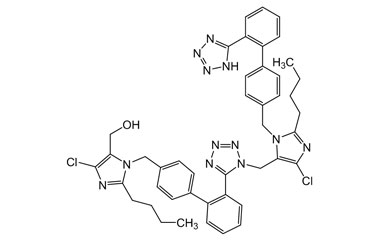
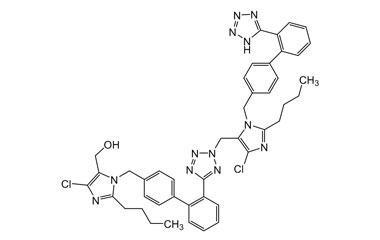
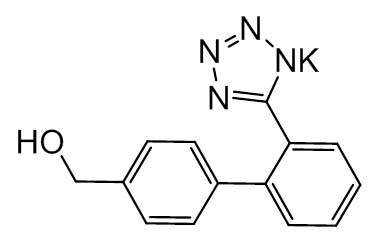
Losartan Potassium (CAS 124750-99-8) Reference Standards and Impurities: Key Elements in Pharmaceutical Testing
Losartan Potassium, a pioneering angiotensin II receptor blocker (ARB), is widely used to treat hypertension and other cardiovascular diseases. Discovered in 1986 and approved by the U.S. Food and Drug Administration (FDA) in 1995, its efficacy and safety rely heavily on stringent quality control. This is achieved through the use of Losartan Potassium (CAS 124750-99-8) reference standards and Losartan Potassium impurities. This article delves into their importance and introduces a reliable solution for supplying these crucial materials that meet the most stringent pharmaceutical standards.
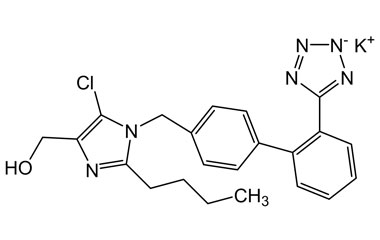
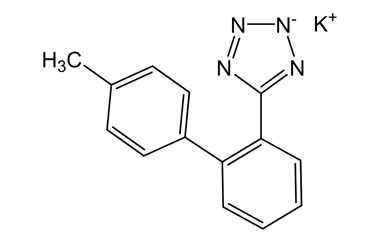
Detailed Information on Losartan Potassium Reference Standard
- Chemical Name: Losartan Potassium
- CAS Number: 124750-99-8
- Molecular Formula: C22H22ClKN6O
- Molecular Weight: 461.00
- Appearance: Crystalline powder
- Storage Condition: 2-8°C (refrigerator)
The Paramount Importance of Losartan Potassium Reference Standards and Impurities
In pharmaceutical manufacturing and testing, quality control is paramount to ensure product safety, efficacy, and regulatory compliance. For Losartan Potassium, the use of pharmaceutical reference standards and Losartan Potassium impurities plays a crucial role in several aspects:
- Qualitative and Quantitative Analysis: Losartan Potassium (CAS 124750-99-8) reference standards accurately determine the Losartan Potassium content in the final product, ensuring correct dosage and treatment efficacy.
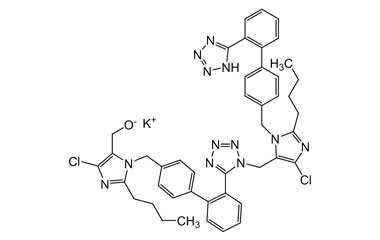
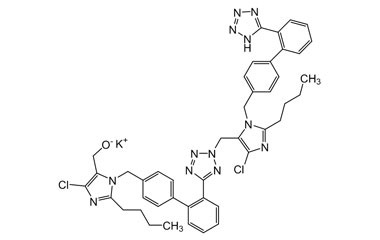
- Purity Control: Losartan Potassium impurities assess the presence and levels of impurities that may arise during synthesis or storage. Impurity control is mandatory to prevent unwanted substances from affecting drug quality and safety.
- Research and Development (R&D): Reference standards and impurities support active substance synthesis, formulation optimization, and stability testing of Losartan Potassium under various conditions.
- International Pharmacopoeia Compliance: Using reference standards and impurities according to pharmacopoeia is mandatory for pharmaceutical manufacturers to comply with strict industry regulations, ensuring market authorization worldwide.
Reliable Source for Losartan Potassium Reference Standards and Impurities
We proudly offer high-quality Losartan Potassium (CAS 124750-99-8) reference standards and Losartan Potassium impurities, meeting the stringent requirements of the pharmaceutical industry. Our product catalog includes Losartan Potassium reference standards, pharmacopoeial and non-pharmacopoeial impurities, and stable isotopes. Specifically:
- Losartan Potassium Reference Standard
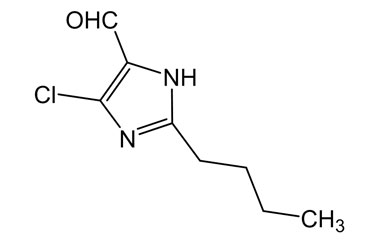
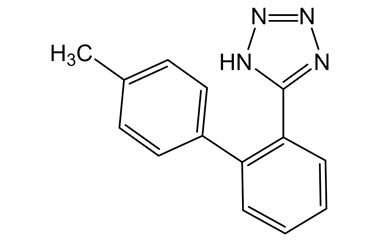
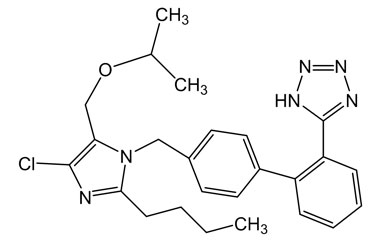
- Pharmacopoeial Impurities of Losartan Potassium: Including impurities listed and controlled by international pharmacopoeias.
- Non-Pharmacopoeial Impurities of Losartan Potassium: Other impurities that may arise but are not officially listed in pharmacopoeias, still requiring control to ensure product safety.
- Stable Isotopes of Losartan Potassium: Isotopic versions of Losartan Potassium used in advanced research, mass spectrometry analysis, and bioavailability testing.
Benefits of Choosing Our Products:
- Guaranteed Quality: All reference standards and impurities undergo rigorous testing, ensuring the highest purity and accuracy, accompanied by a complete certificate of analysis (CoA).
- Product Diversity: Meeting all needs from research and development to production and quality control in pharmaceutical laboratories.
- International Standard Compliance: Our products meet and exceed international standards, facilitating customer compliance with regulations.
- Fast Delivery: With readily available stock, we ensure prompt delivery, preventing disruption to your production and testing processes.
- In-depth Technical Support: Our experienced team is ready to provide dedicated technical advice and support, ensuring optimal product use.
- Optimal Storage and Shipping: Products are stored under ideal conditions (2-8°C) and shipped under standard conditions to maintain the best quality from warehouse to customer.
Contact Us to Enhance Your Pharmaceutical Quality
Investing in high-quality Losartan Potassium (CAS 124750-99-8) reference standards and Losartan Potassium impurities is not only mandatory but also crucial for ensuring the safety and efficacy of pharmaceutical products. Let us be your trusted partner in quality control. Contact us today for a quote and detailed advice on our reference standards and Losartan Potassium impurities – a reliable solution for every pharmaceutical laboratory and company.


 Tiếng Việt
Tiếng Việt