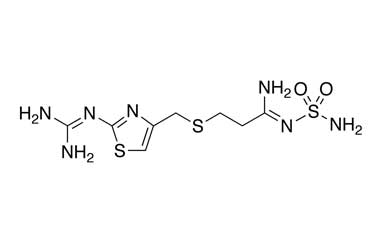
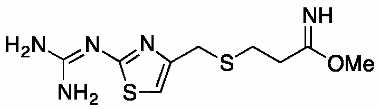
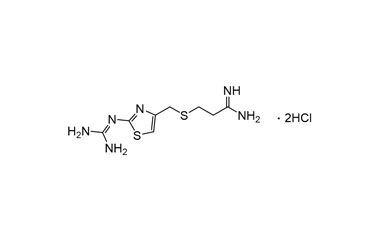
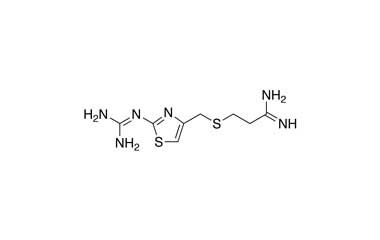
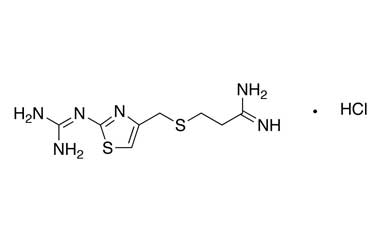
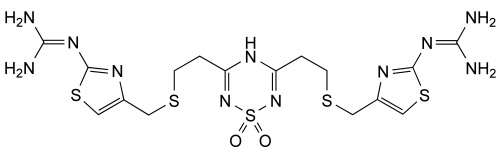
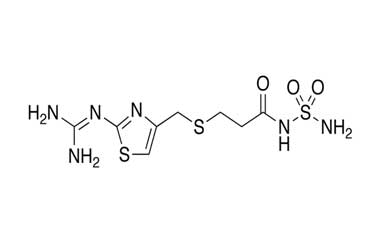
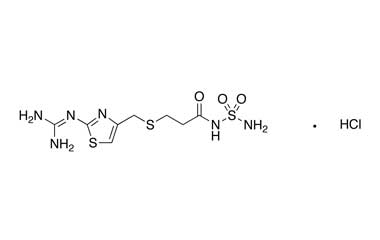
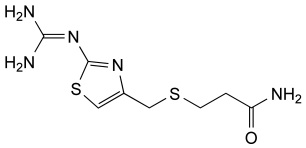
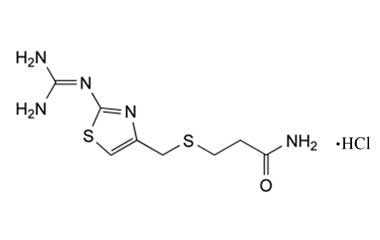
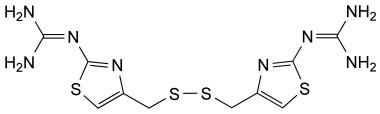
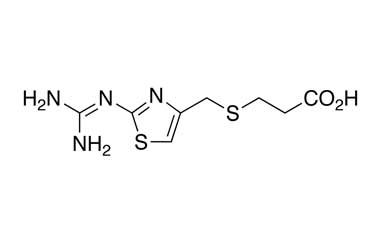
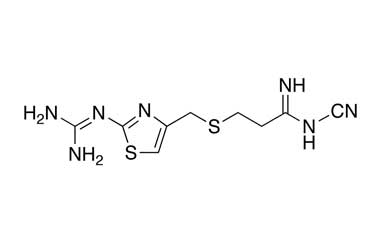
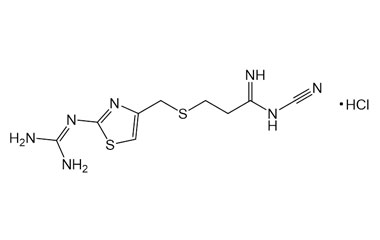
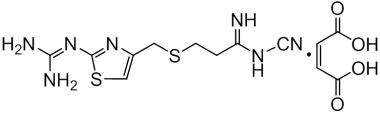
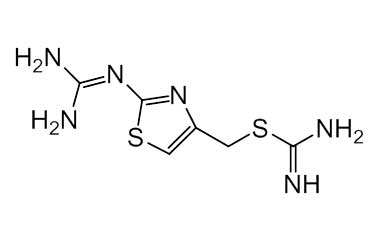
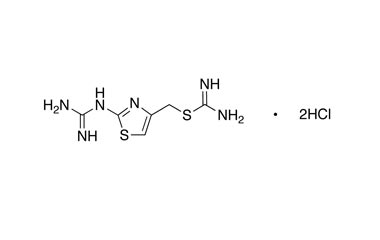
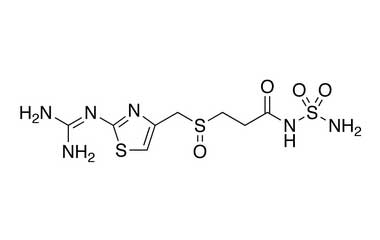
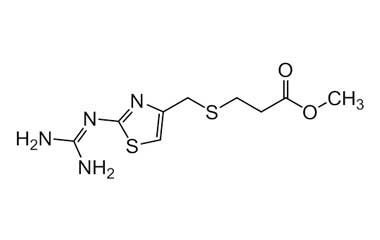
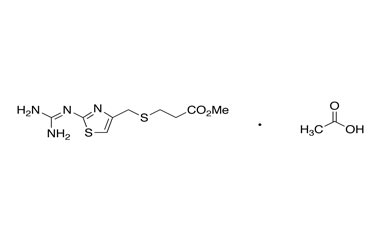
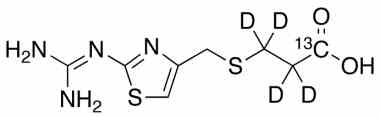
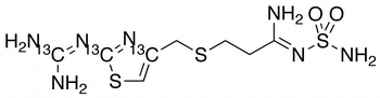
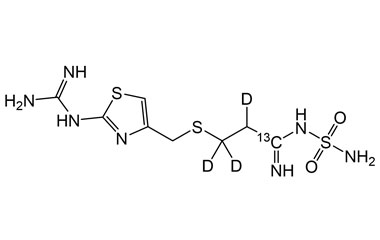
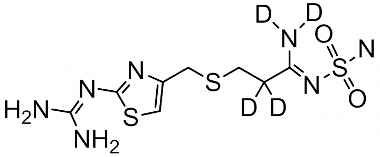
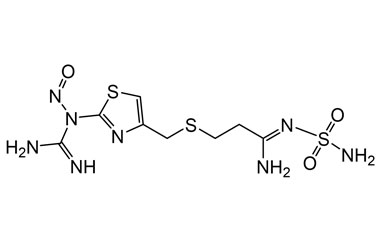
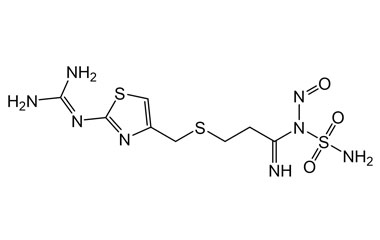
Famotidine Reference Standard (CAS 76824-35-6) and Impurities: Key Elements in Pharmaceutical Testing
Famotidine, an H2 receptor antagonist, is one of the most commonly prescribed drugs for treating acid-related diseases. It is used to treat gastric ulcers, duodenal ulcers, gastroesophageal reflux disease (GERD), and Zollinger-Ellison syndrome. Famotidine was first approved for medical use in 1986 and quickly became a mainstay in gastroenterology due to its efficacy and safety. To ensure the efficacy and absolute safety of the drug, strict quality control through Famotidine reference standards (CAS 76824-35-6) and Famotidine impurities is extremely essential. This article will delve into the importance of Famotidine reference standards and impurities and introduce a solution for supplying reputable reference standards and impurities that meet the most stringent standards of the pharmaceutical industry.
Detailed Information on Famotidine Reference Standard
- Chemical Name: Famotidine
- CAS Number: 76824-35-6
- Molecular Formula: C8H15N7O2S3
- Molecular Weight: 337.44
- Appearance: Crystalline powder or almost white powder
- Storage Condition: 2-8°C (refrigerator)
Outstanding Importance of Famotidine Reference Standards and Impurities
In the field of pharmaceutical manufacturing and testing, quality control is a key factor in ensuring the safety, efficacy, and legal compliance of the product. For Famotidine, the use of pharmaceutical reference standards and Famotidine impurities plays a crucial role in many aspects:
- Qualitative and Quantitative Analysis: Famotidine Reference Standard (CAS 76824-35-6) helps accurately determine the Famotidine content in the final product, ensuring the correct dosage and treatment efficacy.
- Purity Control: Famotidine impurities help assess the presence and level of impurities that may arise during synthesis or storage. Impurity control is mandatory to prevent unwanted substances from affecting the quality and safety of the drug.
- Research and Development (R&D): Reference standards and impurities support the process of active substance synthesis, formula optimization, and testing the stability of Famotidine under various conditions.
- Ensuring Compliance with International Pharmacopoeias: The use of reference standards and impurities according to pharmacopoeias is a mandatory requirement for pharmaceutical manufacturers to comply with the industry’s stringent regulations, thus ensuring that the product is allowed to circulate in the global market.
Reliable Source of Famotidine Reference Standards and Impurities
We are proud to be a reputable supplier of high-quality Famotidine reference standards (CAS 76824-35-6) and Famotidine impurities, fully meeting the stringent requirements of the pharmaceutical industry. Our product portfolio includes Famotidine reference standards, pharmacopoeial and non-pharmacopoeial impurities, as well as stable isotopes. Specifically:
- Famotidine Reference Standard
- Pharmacopoeial Impurities of Famotidine: Including impurities listed and controlled according to international pharmacopoeias.
- Non-Pharmacopoeial Impurities of Famotidine: Other impurities that may arise but are not yet officially listed in the pharmacopoeia, still need to be controlled to ensure product safety.
- Stable Isotopes of Famotidine: Isotopic versions of Famotidine used in in-depth research, mass spectrometry analysis, and bioavailability testing.
Benefits of choosing our products:
- Guaranteed Quality: All reference standards and impurities are rigorously tested to ensure the highest purity and accuracy, accompanied by a complete Certificate of Analysis (CoA).
- Wide Range of Products: Meets all needs from research, development to production and quality control in pharmaceutical laboratories.
- Compliance with International Standards: Our products meet and exceed international standards, making it easy for customers to comply with regulations.
- Fast Delivery: With readily available inventory, we are committed to fast delivery, ensuring that your production and testing processes are not interrupted.
- In-depth Technical Support: Our team of experienced experts is always ready to provide dedicated technical advice and support, ensuring that customers use the products most effectively.
- Optimal Storage and Transportation: Products are stored under ideal conditions (2-8°C) and transported under standard conditions to maintain the best quality from warehouse to customer.
Contact Us Now to Enhance Your Pharmaceutical Quality
Investing in high-quality Famotidine Reference Standard (CAS 76824-35-6) and Famotidine impurities is not only a mandatory requirement but also a key factor in ensuring the safety and efficacy of pharmaceutical products. Let us be your trusted partner in your quality control journey.
Contact us now for a quote and detailed advice on reference standards and Famotidine impurities – a reliable solution for all pharmaceutical laboratories and businesses.


 Tiếng Việt
Tiếng Việt