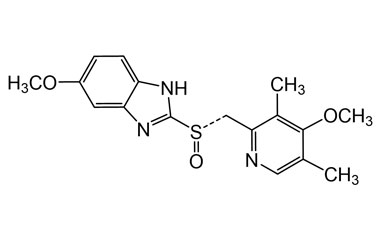
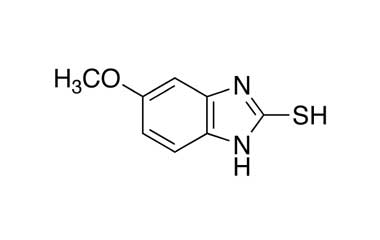
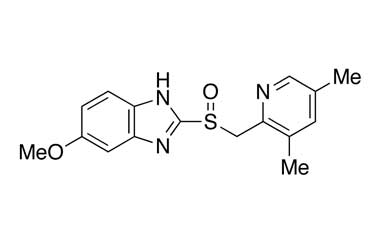
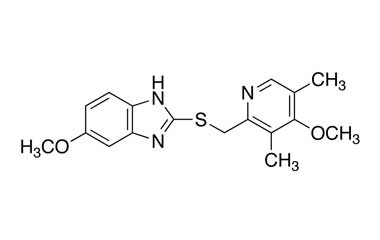
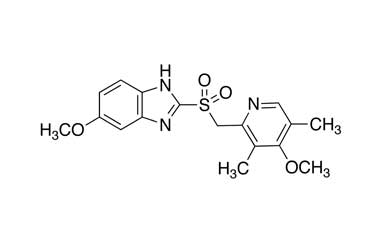
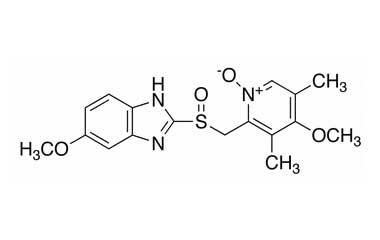
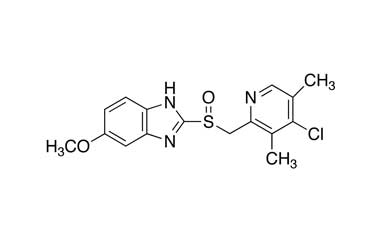
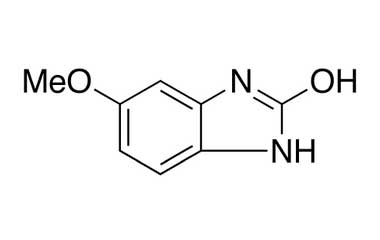
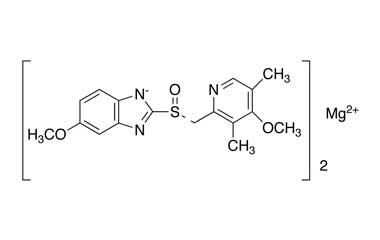
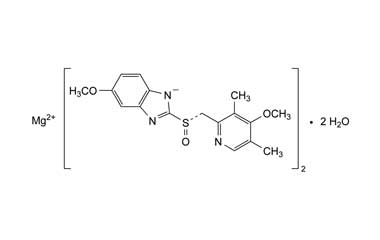
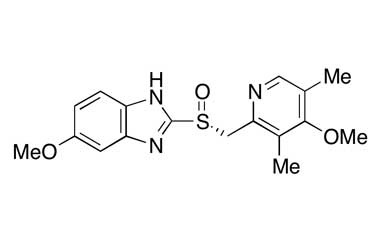
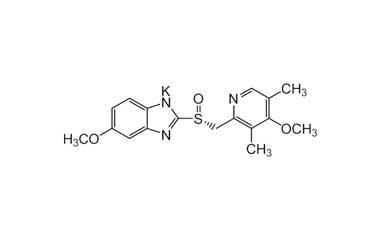
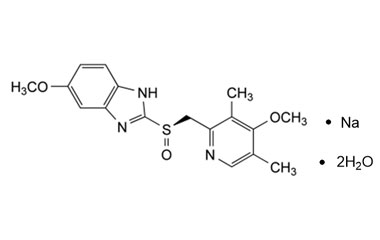
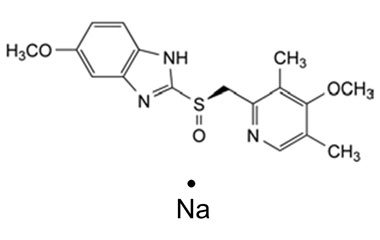
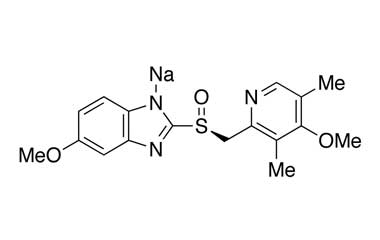
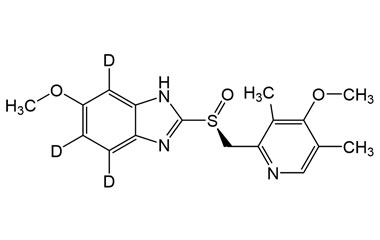
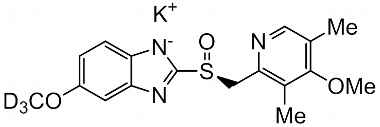
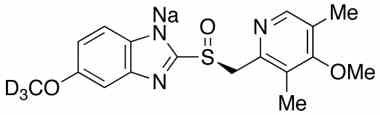
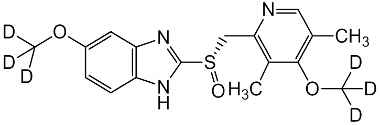
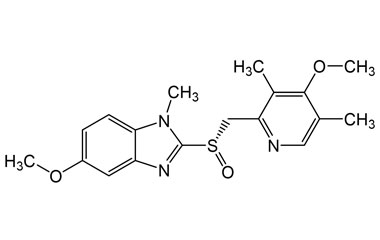
Esomeprazole Standard (CAS 119141-88-7) and Impurities: Key Factors in Pharmaceutical Testing
Esomeprazole, a proton pump inhibitor (PPI), is increasingly affirming its indispensable role in the healthcare field. It is used to treat diseases related to stomach acid such as gastroesophageal reflux disease (GERD), gastric ulcers, and Zollinger-Ellison syndrome. Esomeprazole was patented in 1993 and first approved for medical use in 2000. To ensure the treatment efficacy and absolute safety of the drug, strict quality control through Esomeprazole standards (CAS 119141-88-7) and Esomeprazole impurities is extremely essential. This article will delve into the importance of Esomeprazole standards and impurities and introduce a solution for providing reputable standards that meet the most stringent standards of the pharmaceutical industry.
Detailed Information on Esomeprazole Standard
- Chemical Name: Esomeprazole
- CAS Number: 119141-88-7
- Molecular Formula: C17H19N3O3S
- Molecular Weight: 345.42
- Synonyms: Esomeprazol, Ésoméprazole
- Form: Solid powder
- Storage Conditions: 2-8°C (refrigerator)
Outstanding Importance of Esomeprazole Standards and Impurities
In the field of pharmaceutical production and testing, quality control is a key factor in ensuring the safety, efficacy, and legal compliance of the product. For Esomeprazole, the use of pharmaceutical reference standards and Esomeprazole impurities plays a crucial role in many aspects:
- Qualitative and Quantitative Analysis: Esomeprazole Standard (CAS 119141-88-7) helps to accurately determine the content of Esomeprazole in the final product, ensuring the correct dosage and treatment efficacy.
- Purity Control: Esomeprazole impurities help assess the presence and content of impurities that may arise during synthesis or storage. Impurity control is mandatory to prevent unwanted substances from affecting the quality and safety of the drug.
- Research and Development (R&D): Standards and impurities support the active ingredient synthesis process, formula optimization, and stability testing of Esomeprazole under different conditions.
- Ensuring Compliance with International Pharmacopoeia: The use of standards according to the pharmacopoeia is mandatory for pharmaceutical manufacturers to comply with the strict regulations of the industry, thereby ensuring that the product is allowed to circulate in the global market.
Reliable Source of Esomeprazole Standards and Impurities
We are proud to be a reputable supplier of high-quality Esomeprazole standards (CAS 119141-88-7) and Esomeprazole impurities, fully meeting the stringent requirements of the pharmaceutical industry. Our product catalog includes Esomeprazole reference standards, pharmacopoeial and non-pharmacopoeial impurities, and stable isotopes. Specifically:
- Esomeprazole Standard
- Esomeprazole Pharmacopoeial Impurities: Includes impurities listed and controlled according to international pharmacopoeias.
- Esomeprazole Non-Pharmacopoeial Impurities: Other impurities that may arise but are not officially listed in the pharmacopoeia, still need to be controlled to ensure product safety.
- Esomeprazole Stable Isotopes: Isotopic versions of Esomeprazole used in in-depth research, mass spectrometry analysis, and bioavailability testing.
Benefits of choosing our products:
- Guaranteed Quality: All standards and impurities are rigorously tested, ensuring the highest purity and accuracy, accompanied by a complete Certificate of Analysis (CoA).
- Diverse Product Range: Meets all needs from research and development to production and quality control in pharmaceutical laboratories.
- Compliance with International Standards: Our products meet and exceed international standards, making it easy for customers to comply with regulations.
- Fast Delivery: With readily available inventory, we are committed to fast delivery, ensuring your production and testing processes are not interrupted.
- In-depth Technical Support: Our experienced team of experts is always ready to provide dedicated technical advice and support, ensuring customers use the product most effectively.
- Optimal Storage and Transportation: Products are stored under ideal conditions (2-8°C) and shipped under standard conditions to maintain the best quality from warehouse to customer.
Contact Us Now to Upgrade Your Pharmaceutical Quality
Investing in high-quality Esomeprazole standards (CAS 119141-88-7) and Esomeprazole impurities is not only a mandatory requirement but also a key factor in ensuring the safety and efficacy of your pharmaceutical products. Let us be your trusted partner in your quality control journey.
Contact us now for a quotation and detailed advice on standards and Esomeprazole impurities – a reliable solution for all pharmaceutical laboratories and businesses.


 Tiếng Việt
Tiếng Việt