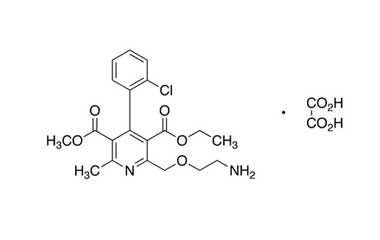
Amlodipine Reference Standard (CAS 88150-42-9) and Related Impurity Standards: Essential Components in Pharmaceutical Quality Control
Amlodipine, a widely used medication for the treatment of hypertension and angina, has firmly established its essential role in modern therapeutics. First approved by the FDA in 1987, Amlodipine is a well-recognized antihypertensive agent classified as a dihydropyridine calcium channel blocker. To ensure its therapeutic efficacy and patient safety, stringent quality control measures are required—particularly through the use of the Amlodipine Reference Standard (CAS 88150-42-9) and related impurity standards. This article presents a comprehensive solution for sourcing high-quality reference materials that comply with the most rigorous standards of the pharmaceutical industry.
Detailed information about Amlodipine Reference Standard:
- Chemical name: Amlodipine
- CAS number: 88150-42-9
- Molecular formula: C20H25ClN2O5
- Molecular weight: 408.88
- Synonyms: 2-[(2-Aminoethoxy)methyl]-4-(2-chlorophenyl)-3-ethoxycarbonyl-5-methoxycarbonyl)-6-methyl-1,4-dihydropyridine; (R,S)-Amlodipine; Amlopres; Intervask; Pelmec; Egiramlon; Racemic Amlodipine; Adipin; (±)-Amlodipine; Sofvasc (USP)
- Classification: Intermediate, heterocyclic, pharmaceutical standard, aromatic compound, pure chemical, dihydropyridine
- Form: Off-white to pale yellow solid
- Storage conditions: 2-8°C (refrigerated)
The Importance of Amlodipine Reference Standards and Impurities
In the pharmaceutical manufacturing and testing industry, quality control is a key factor in ensuring product safety, efficacy and regulatory compliance. For Amlodipine, the use of pharmaceutical standards and Amlodipine impurity standards is extremely important in many aspects:
- Qualitative and Quantitative Analysis: Amlodipine standards (CAS 88150-42-9) help to accurately determine the Amlodipine content in the final product, ensuring correct dosage and therapeutic efficacy.
- Purity Control: Amlodipine impurity standards help to assess the presence and content of impurities that may arise during synthesis or storage. Impurity control is mandatory to prevent unwanted substances from affecting the quality and safety of the drug.
- Research and Development (R&D): Reference materials and standards support the synthesis of active ingredients, optimize formulations, and test the stability of Amlodipine under various conditions.
- Ensuring International Pharmacopoeia Compliance: The use of reference materials and standards according to pharmacopoeias is a mandatory requirement for pharmaceutical manufacturers to comply with strict industry regulations, thereby ensuring that their products are allowed to circulate in the global market.
A Reliable Source for Amlodipine Reference Standards and Impurites
We are proud to be a reputable supplier of Amlodipine Reference Standards (CAS 88150-42-9) and high quality Amlodipine Impurites standards, fully meeting the stringent requirements of the pharmaceutical industry. Our product portfolio includes Amlodipine API reference standards, and pharmacopoeial and non-pharmacopoeial impurities, as well as stable isotopes. Specifically:
- Amlodipine reference standard form
- Amlodipine pharmacopoeial impurities: Including impurities listed and controlled by international pharmacopoeias such as EP, BP.
- Amlodipine non-pharmacopoeial impurities: Other impurities that may arise but are not officially listed in the pharmacopoeia, still need to be controlled to ensure product safety.
- Amlodipine stable isotopes: Isotopic versions of Amlodipine are used in in-depth studies, mass spectrometry analysis and bioavailability testing.
Benefits of choosing our products:
- Fast delivery: Goods are always ready to be delivered to you.
- Quality assurance: All standards and impurities are strictly tested, ensuring the highest purity and accuracy.
- Diversity of products: Meet all needs from research to production and quality control.
- Comply with international standards Standards: Our products meet international standards and pharmacopoeias.
- Technical support: Our team of experienced experts is always ready to advise and support customers.
- Optimal storage and transportation: Products are stored at 2-8°C and transported under standard conditions to maintain the best quality.
Contact Now to Improve the Quality of Your Pharmaceuticals
Investing in high-quality Amlodipine Reference Standards (CAS 88150-42-9) and high quality Amlodipine Impurites standards is not only a mandatory requirement but also a key factor to help you ensure the safety and effectiveness of your pharmaceutical products. Let us be your trusted partner in your quality control journey.
Contact us now to receive a quote and detailed advice on Amlodipine standard products and standards – a reliable solution for all laboratories and pharmaceutical businesses.


 Tiếng Việt
Tiếng Việt